Are you ready for the new Machinery directive 2006/42/ec?
Let Wellkang be your professional CE Marking EC/EU Authorised/Authorized representative under new Machinery directive 2006/42/ec!
Our service varies greatly from case to case, from product to product. Please click here now for a quotation.
On 29 December 2009, the new Machinery Directive 2006/42/EC will replace the currently valid Machinery Directive 98/37/EC. The 2006/42/EC will regulate the placing on the market, and the putting into service, of machinery in the European Economic Area (EEA), as currently undertaken by Directive 98/37/EC.
- In general, the new Machinery Directive 2006/42/EC follows the same principles as the old directive 98/37/EC. The changes are largely a question of tidying up acknowledged flaws in the old directive and addressing some of the difficulties that have been experienced in making it work properly in practice.
- The new Machinery Directive 2006/42/EC aims at providing greater clarity than the old directive 98/37/EC, e.g. with a new definition of the core concept of ‘machinery’ and in the dividing lines between itself and the Lifts and Low Voltage Directives.
- Another significant change is the introduction of a quality assurance conformity assessment module as an option for relevant manufacturers. Some of the other changes will be described below.
Extensive changes are especially prevalent with the conformity assessment procedure of Annex IV Machines. In the event that there are no harmonized EU standards or the product was not constructed under compliance of these standards, an EC Type Examination by a recognized test center is currently required. The new directive now opens up the option of self-certification for the manufacturer without the participation of a test center, if they have a quality assurance procedure in accordance with Annex X.
Further changes are:
- The basic safety and health requirements (Annex I) will in future require a risk assessment by the manufacturer.
- In the old Machinery Directive there are different procedures for proving the safety of machinery, exchangeable equipment, safety components, chains/ropes/belts for lifting purposes, cardan shafts and load-carrying equipment. In the future the same machine regulations will also apply for these products. They will have to be distributed in the future with CE conformity assessment, declaration of conformity and the required user information.
- The requirements for "part-machines" (also referred to as "incomplete machines") have been re-formulated in the new version of the Machinery Directive. Until now a manufacturer declaration was sufficient, but in the future the manufacturer will also have to supply a declaration of incorporation, which must specify which requirements of the directive apply to the part-machine and have been complied with. Installation instructions must be provided with the machine’s documentation.
- Lifting devices with a speed of up to 0.15 m/s of the load carrier are subject to the Machinery Directive; with a speed of more than 0.15 m/s they are subject to the Lift Directive (if they are not covered by its rules of exception).
- The delimitation of the Low Voltage Directive is no longer regulated as risk-related, but rather product-related.
- Construction site lifts are subject to the Machinery Directive.
- Instead of a "hazard analysis" a "risk assessment" and "risk evaluation" are required.
- Clearer delimitation of the Machinery Directive for the Low Voltage Directive.
- Internal production controls for series machines (Annex VIII).
- The validity of EC Type Examination certifications must be checked by the test center every 5 years. Manufacturers and test centers are obligated to retain the relevant technical documents for 15 years.
CE marking
- The CE conformity marking shall consist of the initials ‘CE’ taking the following form:
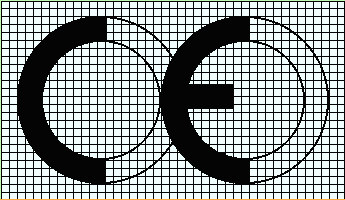
- If the CE marking is reduced or enlarged the proportions shown in the above drawing must be respected.
- The various components of the CE marking must have substantially the same vertical dimension, which may not be less
than 5 mm. The minimum dimension may be waived for small-scale machinery.
- The CE marking must be affixed in the immediate vicinity of the name of the manufacturer or his authorised representative, using the same technique.
- Where the full quality assurance procedure referred to in Article 12(3)(c) and 12(4)(b) has been applied, the CE marking
must be followed by the identification number of the notified body.
Technical File for Machinery
The Technical File must demonstrate that the machinery complies with the requirements of this Directive. It must cover the design, manufacture and operation of the machinery to the extent necessary for this assessment. The Technical File must be compiled in one or more official Community languages, except for the instructions for the machinery, for which the special provisions of
Annex I, section 1.7.4.1 apply.
- The Technical File shall comprise the following:
- (a) a construction file including:
- a general description of the machinery,
- the overall drawing of the machinery and drawings of the control circuits, as well as the pertinent
descriptions and explanations necessary for understanding the operation of the machinery,
- full detailed drawings, accompanied by any calculation notes, test results, certificates, etc., required to
check the conformity of the machinery with the essential health and safety requirements,
- the documentation on risk assessment demonstrating the procedure followed, including:
(i) a list of the essential health and safety requirements which apply to the machinery,
(ii) the description of the protective measures implemented to eliminate identified hazards or to reduce
risks and, when appropriate, the indication of the residual risks associated with the machinery,
- the standards and other technical specifications used, indicating the essential health and safety requirements
covered by these standards,
- any technical report giving the results of the tests carried out either by the manufacturer or by a body
chosen by the manufacturer or his authorised representative,
- a copy of the instructions for the machinery,
- where appropriate, the declaration of incorporation for included partly completed machinery and the
relevant assembly instructions for such machinery,
- where appropriate, copies of the EC declaration of conformity of machinery or other products incorporated
into the machinery,
- a copy of the EC declaration of conformity;
- (b) for series manufacture, the internal measures that will be implemented to ensure that the machinery remains
in conformity with the provisions of this Directive.
The manufacturer must carry out necessary research and tests on components, fittings or the completed
machinery to determine whether by its design or construction it is capable of being assembled and put into
service safely. The relevant reports and results shall be included in the Technical File.
- The Technical File referred to in point 1 must be made available to the competent authorities of the Member
States for at least 10 years following the date of manufacture of the machinery or, in the case of series manufacture,
of the last unit produced.
The Technical File does not have to be located in the territory of the Community, nor does it have to be permanently
available in material form. However, it must be capable of being assembled and made available within a
period of time commensurate with its complexity by the person designated in the EC declaration of conformity.
The Technical File does not have to include detailed plans or any other specific information as regards the subassemblies
used for the manufacture of the machinery unless a knowledge of them is essential for verification of
conformity with the essential health and safety requirements.
- Failure to present the Technical File in response to a duly reasoned request by the competent national authorities
may constitute sufficient grounds for doubting the conformity of the machinery in question with the essential
health and safety requirements.
Relevant Technical Documentation
for Partly Completed Machinery
This part describes the procedure for compiling relevant technical documentation. The documentation must show
which requirements of this Directive are applied and fulfilled. It must cover the design, manufacture and operation
of the partly completed machinery to the extent necessary for the assessment of conformity with the essential
health and safety requirements applied. The documentation must be compiled in one or more official Community
languages.
It shall comprise the following:
- (a) a construction file including:
- the overall drawing of the partly completed machinery and drawings of the control circuits,
- full detailed drawings, accompanied by any calculation notes, test results, certificates, etc., required to check
the conformity of the partly completed machinery with the applied essential health and safety requirements,
- the risk assessment documentation showing the procedure followed, including:
- (i) a list of the essential health and safety requirements applied and fulfilled,
- (ii) the description of the protective measures implemented to eliminate identified hazards or to reduce
risks and, where appropriate, the indication of the residual risks,
- (iii) the standards and other technical specifications used, indicating the essential health and safety requirements
covered by these standards,
- (iv) any technical report giving the results of the tests carried out either by the manufacturer or by a body
chosen by the manufacturer or his authorised representative,
- (v) a copy of the assembly instructions for the partly completed machinery;
- (b) for series manufacture, the internal measures that will be implemented to ensure that the partly completed
machinery remains in conformity with the essential health and safety requirements applied.
The manufacturer must carry out necessary research and tests on components, fittings or the partly completed
machinery to determine whether by its design or construction it is capable of being assembled and used safely. The
relevant reports and results shall be included in the technical file.
The relevant technical documentation must be available for at least 10 years following the date of manufacture of
the partly completed machinery or, in the case of series manufacture, of the last unit produced, and on request
presented to the competent authorities of the Member States. It does not have to be located in the territory of the
Community, nor does it have to be permanently available in material form. It must be capable of being assembled
and presented to the relevant authority by the person designated in the declaration for incorporation.
Failure to present the relevant technical documentation in response to a duly reasoned request by the competent
national authorities may constitute sufficient grounds for doubting the conformity of the partly completed
machinery with the essential health and safety requirements applied and attested.
Why do you need a representative in Europe?
Why must the manufactures of medical devices appoint a EU Authorized Representative?
Why is the Authorized Representative different from the importer/distributor ?
Why choose Wellkang?
How can Wellkang help you?
For more information, please visit our Questions and Answers section.
|


